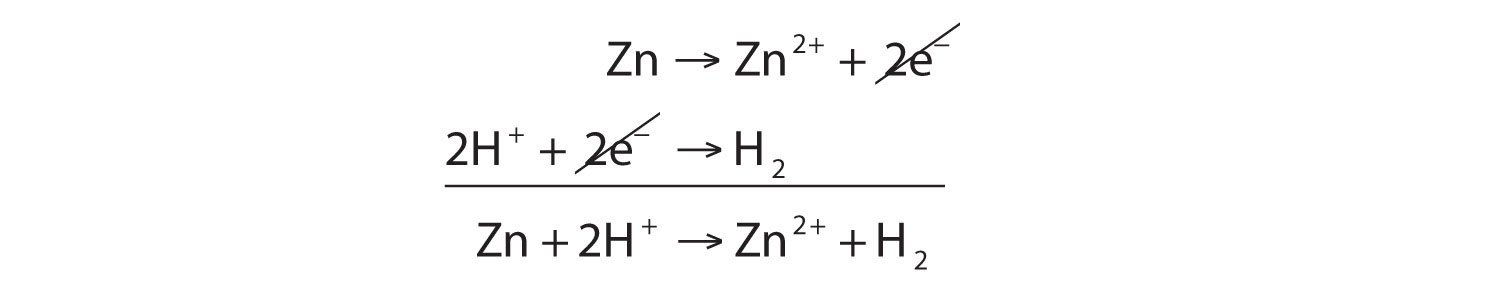Trimetallic Cu–Ni–Zn/H-ZSM-5 Catalyst for the One-Pot Conversion of Levulinic Acid to High-Yield 1,4-Pentanediol under Mild Conditions in an Aqueous Medium | ACS Catalysis

SOLVED: In the reaction Zn + H+ —> Zn2+ + H2, Zn is oxidized and H is reduced. How many electrons would be produced and used in the balanced half-reactions? Complete the
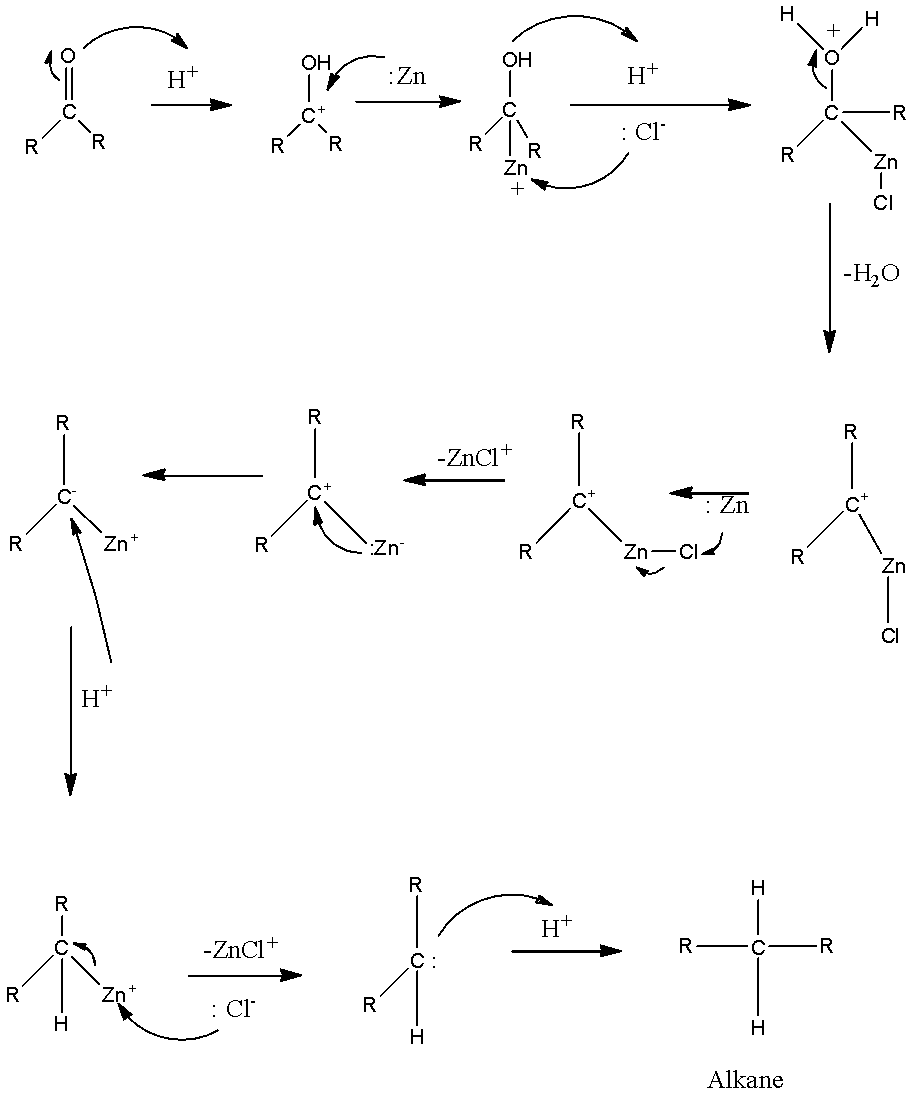
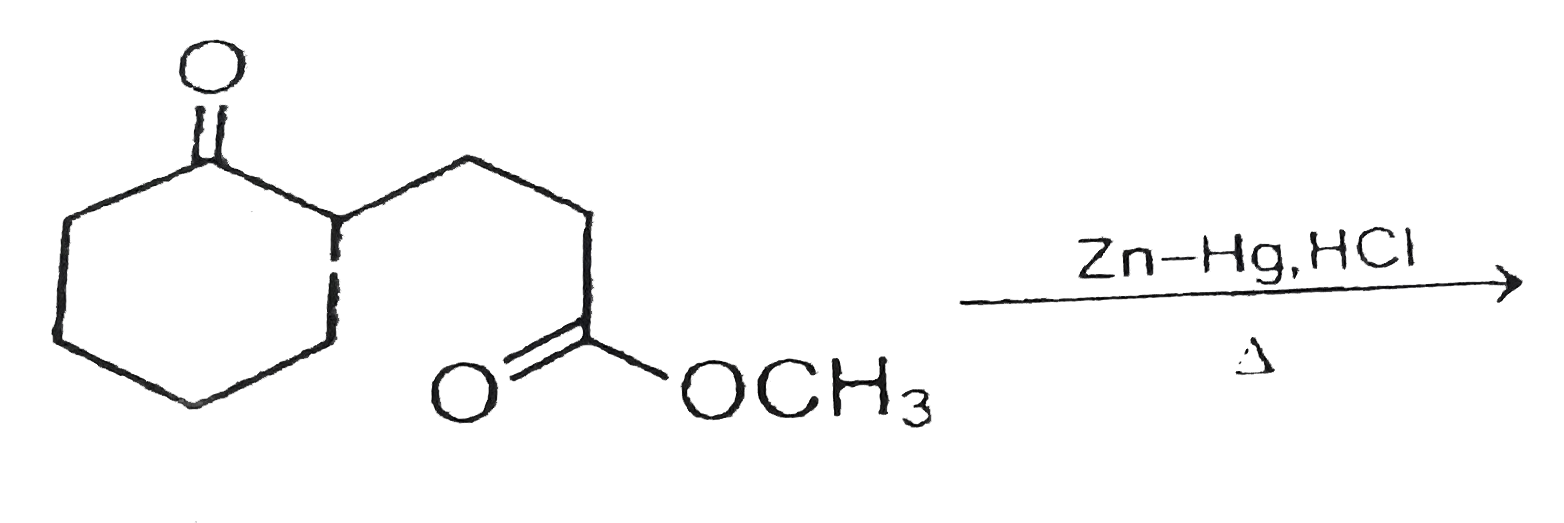
Which of the following is reduced with Zn-Hg and HCl to give alkane?(a).Ethyl acetate(b).Acetic acid(c).Acetamide(d).Butan-2-one

A Comparison of Two Zinc Hydride Catalysts for Terminal Alkyne C–H Borylation/Hydroboration and the Formation of 1,1,1-Triborylalkanes by Tandem Catalysis Using Zn–H and B–H Compounds | Organometallics
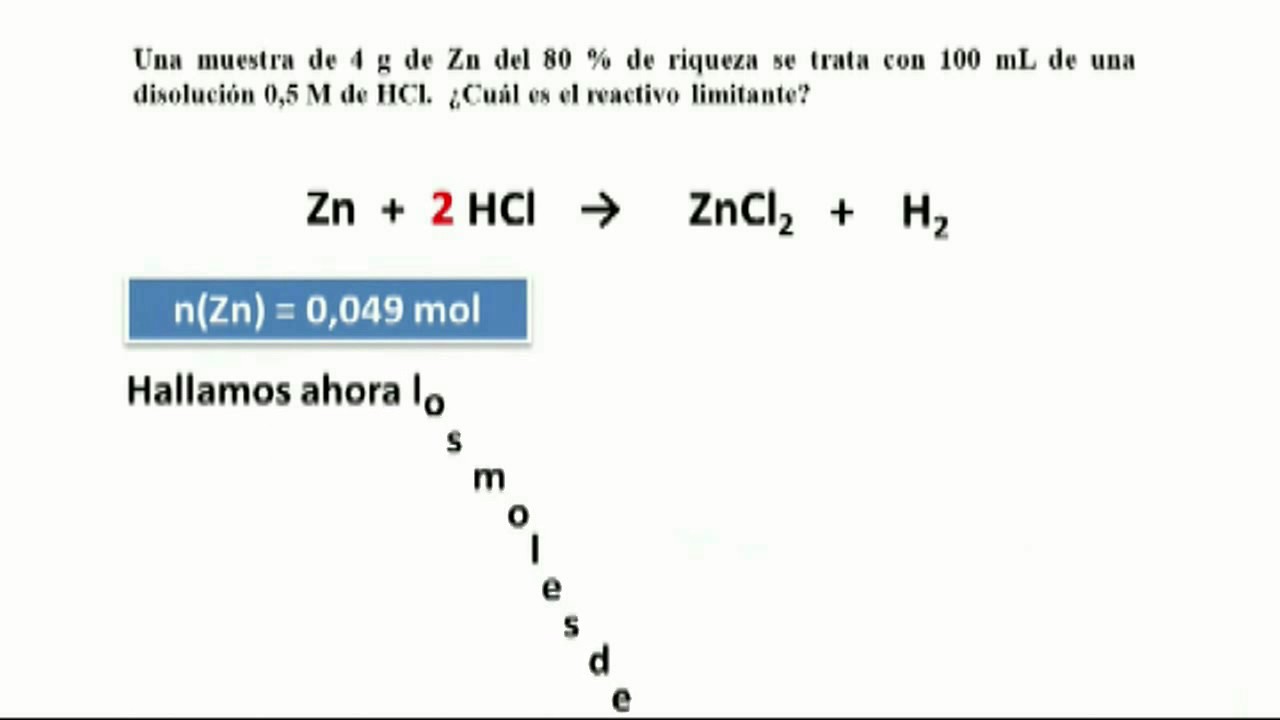
Consider the following reactions (unbalanced) Zn + hot conc. H2SO4 →G + R + X - Sarthaks eConnect | Largest Online Education Community
43. Balance the following equation: Zn + (H+) —> (Zn+2) + H2 (Zinc reacts with hydrogen ion to give Zinc ion and Hydrogen gas.)

How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric Acid + Zinc) | How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric Acid + Zinc) Balancing equations could




![In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is: In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is:](https://dwes9vv9u0550.cloudfront.net/images/7671796/5bdff3d8-c0c9-4fc8-89cd-f9235a0c1b30.jpg)